RERDS – Centre for Pharmaceutical Research (CPR)
Sponsored by Raghavendra Educational and Rural Development Society
(Reg. No. 198/2002)
In view of developing Pharmaceutical Research in institution, RERDS constituted the “Centre for Pharmaceutical Research (CPR)” in the Research and Development Cell (R & D Cell) to offer Research and Training facilities.
Preamble
- Functioning as a private non-profit organization, recognized as SIRO by DSIR in the year 2018.
- In the Year 2010, it was started as Centre for Pharmaceutical Research (CPR) as annex to Raghavendra Institute of Pharmaceutical Education and Research (RIPER), but
- From 2016, it is renamed as RERDS – Centre for Pharmaceutical Research (CPR) and functioning as independent organization with its own decentralized facility, faculty, vision and objectives vide RERDS society resolution.
Research Vision
“Committed to excel in the area of basic and pharmaceutical research, critically to resolve social and public problems through technological innovations and global collaborations”.
Quality Policy
We committed ourselves to shape the basic and pharmaceutical research as translating research and innovations for a better society through global partnership and continuous improvement.
Objectives
- To perform collaborative and translational research and development for delivering new pharmaceuticals / products as preventive / curative ailments against life threatening diseases.
- To undertake collaborative research projects with the goal of a seamless transition of therapeutic molecules to clinical stages and beyond.
- To conduct programme / training relevant to societal needs, advancement in research techniques for the academia and industrial personnel engaged in the Quality Pharmaceutical deliverables.
- To facilitate a platform for the researchers towards their development in innovative research, collaborations, patent filing and publications.
- To frame stable platform with reputed research organization and Universities from India and abroad on productive research and product development on the context of mutual benefits.
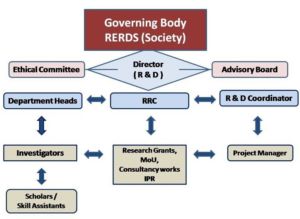
RERDS-CPR – Organization Chart
RERDS-CPR – Research Advisory Board
| Name | Designation | Address |
| Ex-Officio Member | Director R&D | JNTUA, Anantapur |
| Prof. C. Kokate | Ex Vice-Chancellor | KLE University, Belgaum, Karnataka |
| Prof. B. Suresh | Vice-Chancellor | JSS University, Mysore, Karnataka. |
| Prof N Udupa | Professor | Department of Pharmacy Management, Manipal University, Manipal, Karnataka |
| Prof K.B. Chandrasekhar | Vice-Chancellor | Krishna University, Machilipatnam, Andhra Pradesh |
| Dr. Devanna | Director and Professor | JNTUA, Anantapur, Andhra Pradesh |
| Dr G N Sastry | Senior Scientist | Senior Scientist, IICT, Hyderabad, Telangana. |
| Dr Bontha Veera raju babu | Scientist F | Indian Council for Medical Research, New Delhi. |
| Dr U Ramesh | Scientist | Indian Institute of Chemical Technology (IICT), Hyderabad. |
| Dr Azger Dusthackeer VN | Scientist – Bacteriologist | National Institute of Research in Tuberculosis (NIRT), Chennai. |
| Dr KVSR Prasad | Retired Professor | Professor in pharmacology, Mahila University, Tirupati, Andhra Pradesh. |
| Dr A Pramila Rani | Principal | University College of Pharmaceutical Sciences, Acharya Nagarjuna University, Guntur Dist., Andhra Pradesh. |
| Dr Dina Nair | Scientist (Medical) | National Institute of Research in Tuberculosis (NIRT), Chennai |
| Dr CH V Rao | Principal Scientist | Pharmacology Division, National Botanical Research Institute, India |
| Dr Amit Tiwari | Principal Scientist | Cancer Biology, The University of Toledo, USA |
| Dr. Sarangapani | Former Professor | Kakatiya University, Warangal, Telangana. |
| Dr T K Ravi | Principal & Professor | College of Pharmacy, SRIPMS, Coimbatore, Tamil Nadu. |
| Dr H G Shiva Kumar | Principal & Professor | College of Pharmacy, JSS University, JSS University, Mysore, Karnataka. |
Animal Ethical Committee approved by CPCSEA, Govt. of India (Twice in a Year)
| Name and designation | Address | Nominated as |
| Dr. C. Madhava Reddy | Room No 124, Sir CV Ram Science Building, Deptt of Biotechnology and Bioinformatics, Yogi Verma University, Kadapa – 516005, AP
Contact No: 9703599690 Email: cmadhavareddy@gmail.com |
Main Nominee |
| Dr. Thavitiki Prasad Rao, | Assistant Professor, Dept of Veterinary Science, Gopavaram, Proddatur – 516360, Kadapa, Andhra Pradesh.
Contact No: 9866903250 |
Link Nominee |
| Dr. C. Rajaram
|
Memorial College of Pharmacy, 44/35 – 1, Prakruthi Nagar, Utukur, Kadapa – 516003
Contact No: 9642780790 Email: rajaram212121@gmail.com |
Scientist from outside the Institute |
| Dr. A. Hari Krishna | 1 – 68-6, Rajendra Prasad Steet, Kadiri 515591, Ananthapuramu Dist, Andhra Pradesh
Contact No: 9497354033 Email: hkagisam@gmail.com |
Social aware Nominee |
| Dr. Y. Padmanabha Reddy
Principal |
Raghavendra Inst. of Pharm. Education and Research
Email: ypreddyatp@gmail.com |
Scientist from different biological discipline, chairperson (Chairperson) |
| Dr. K. Somasekhar Reddy
Asso. Professor |
Raghavendra Inst. of Pharm. Education and Research
Email:somu.reddyvaru@gmail.com
|
Scientist In-charge of Animal Hose Facility |
| Dr. B Pradeepkumar
Asso. Professor |
Raghavendra Inst. of Pharm. Education and Research
Email:bhupalampradeep@gmail.com
|
Biological Scientist |
| Dr. Vijay R Chidrawar
Professor – Director, R&D |
Raghavendra Inst. of Pharm. Education and Research | Scientist from different discipline |
| Dr. P. Swetha | Raghavendra Inst. of Pharm. Education and Research | Veterinarian |
RERDS-CPR – Research Review Committee
With reference to the promotion of quality research work in the institution, the following members are nominated as members of the reconstituted Research Review Committee with their respective position. This committee shall be effective from 04.04.2022 to till further orders.
| S. No | Name | Designation | Nominated Position |
| 1 | Dr. Y. Padmanabha Reddy | Principal | Chairman |
| 2 | Dr. Vijay R Chidrawar | Director (R&D Division) | Secretary |
| 3 | Dr. M. Vijaya Jyothi | Research Co-ordinator | Coordinator |
| 4 | Dr. V. Uma Maheshwara Rao | Director (Academics) | Member |
| 5 | Dr. H. Abdul Ahad | Professor | Member |
| 6 | Dr. Nawaz Mohammed | Asso. Professor | Member |
| 7 | Dr. C. Haranath | Asso. Professor | Member |
| 8 | Dr. K. Somasekhar Reddy | Asso. Professor | Member |
| 9 | Dr. K. Vinod Kumar | Asso. Professor | Member |
| 10 | Dr. B. Pradeep Kumar | Asso. Professor | Member |
| 11 | Dr. Vinod Kumar Nelson | Asso. Professor | Member |
Note:
- The above committee shall also be acting as Doctoral Committee for the review of PhD works / or any research matters / whenever required by the Principal / University.
- Research Advisory Board (RAB) shall be assisting the RRC, whenever required.
- Meetings and resolutions can be made when 70% of members are present.
Various Units / Research Division under RERDS-CPR
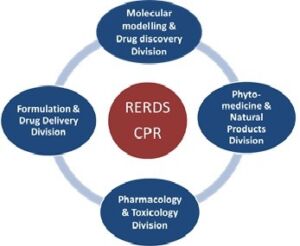
Research Team
| Name & Qualification | Designation |
| Dr. Y. Padmanabha Reddy., M. Pharm., PhD., FIC | President / CEO, RERDS – CPR |
| Dr. Vijay R Chidrawar., M. Pharm., PhD | Director, RERDS – CPR |
| Dr. K. Somasekhar Reddy., M. Pharm., PhD | Head, Pharmacology & Toxicology Division |
| Dr. M. Vijaya Jyothi, M. Pharm., PhD | Head, Molecular Modelling & Drug Discovery Division |
| Dr. Vinod Kumar Nelson., M. Pharm., PhD | Head, Phytomedicine & Phytochemistry Division |
| Dr. Hindustan Abdul Ahad, M. Pharm., PhD., FAGE | Head, Formulation & Drug Delivery Division |
| Dr. B. Pradeep Kumar., M. Pharm., PhD | Scientist, Pharmacological screening and animal ethical clearance |
| Mr. C. Naresh Babu., M. Pharm., | Research Associate |
| Mr. E. Bhargav, M. Pharm., | Research Associate |
| Mrs. B. Nagasubha, M. Pharm., | Research Associate |
| Mr. P. Chiranjeevi, M. Pharm., | Research Associate |
| Mrs. S. Triveni, M. Pharm., | Research Associate |
| Mrs. Y. Sravani, M. Pharm., | Research Associate |
| Mr. S. Mahaboob Basha., B.Sc, D. Pharm., | Purchase and Store keeping |
Consultancy /Internal Revenue Policy with reference to the promotion of consultancy works, the following are the framed internal revenue / Consultancy Policies, till further notice.
- There should be letter from customer (Researchers /PhD scholars/Industry/Institution) addressed to the principal.
- The Principal shall forward the letter to R & D director.
- R&D Division shall fix the charges as per our norms in consultation with Heads.
- Then copy of the letter and requirement shall be forwarded to the Concern Heads. Then Head shall allot the work to Skill assistants.
- Once the report is ready, the duly signed report shall be forwarded to R&D.
- The Report will be dispatched / issued to applicant, once the payment is cleared.
- All the details of Reports / amount will be entered in register / duly counter signed by principal.
- The copy of facility request letter shall also be documented in Concern department.
Term & Conditions
- There should not be any hidden work /unethical report to customer without the knowledge of Head & R&D Director. In such cases, serious action will be executed at the discretion of Principal.
- If any faculty/Scholars invite consultancy work, 10% of the total cost charged will paid as Reward.
Research Activity – Ethical Clearance Procedure
The following shall be the procedure for conducting experiments on Animals /Human subjects,
- Study involving Human /Animal subjects should not be initiated until the ethical clearance is issued by R&D Division.
- There shall be only one Animal ethical committee meeting and One Human Ethical Review Board meeting during the one academic year (July 1st to June 31st).
- However, in case of emergency, the protocol may be mailed to Nominee and the decision is at the discretion of Chairman and Govt. Nominee.
- During Ethical clearance meeting, the principal investigator / Co-investigator / Students must be available to defend the queries.
- All investigators must give proposal presentation and submit the copy of PPT for signing along with the prescribed format of application.
- All UG Projects / experiments should be designed in such a way that no animal is allowed to sacrifice.
- Animals will be allowed to dissect only in PG / Granted Projects.
- The copy of animal ethical committee approval letter must be submitted to pharmacology department to touch /use animals.
- In case of human subjects, interventions should be as per the directions of ethical committee and should not violate GLP / GCP.
Indent of chemicals / supply of chemicals to Research
(Applicable for: M. Pharm / B. Pharm Projects, Grants)
With reference to the promotion of research works, the following are the framed Policies, till further notice.
| Step 1 | A student / scholar shall prepare a list of chemicals for their research along with the research title and protocol number (if) and get signed by their supervisor |
| Step 2 | The signed letter shall be forward to the R&D through HOD with remarks, HOD should write the comments on chemicals if it is project specific, and its importance/ if belongs to consultancy/funded. |
| Step 3 | The R&D division will forward the letter with the comment to the concern section through Principal / Academic coordinator. The copy the letter shall be retailed by the HOD. |
| Step 4 | The HOD shall ask concern lab assistant to submit the Indent for the same in prescribed format. |
| Step 5 | The Lab assistant shall follow up with store keeper for placing and procuring chemicals. |
| Step 6 | Once the chemicals are received from the store, Lab assistant shall enter in their department stock resister / distribution register. |
| Step 7 | Then chemicals shall be supplied to students /scholars, Student shall hold the responsibility of submitting the unused chemicals to lab assistant with utilization record /job record. |
NOTE:
- No students are allowed in the purchase and permission process.
- If any project specific chemicals, R&D and HOD shall decide to supply or not to supply.
- In case research/project belongs to consultancy / UGC/AICTE /etc funded, all chemicals shall be supplied by the institute Plagiarism Policy.
| Applicable to | B. Pharm Project book, |
| M. Pharm Dissertation book, | |
| Pharm D Clerkship book & Internship book | |
| PhD thesis, | |
| Reference | As required by JNTUA, other Regulatory systems |
| % Plagiarism allowed | B. Pharm (up to 40%) |
| M. Pharm / Pharm D as per JNTUA Norms (up to 30%) | |
| For PhD it may change depends on university. |
Procedure:
- With reference to the above cited information regarding plagiarism policy, I request all faculty members TO SIGN THE RESEARCH REPORT only after obtaining plagiarism report from R&D Division, RIPER.
- The student/Scholar shall submit the document (word document) in a USB /Pen-drive to R&D COORDINATOR for plagiarism report. See that the document is EXCLUDED from contents like, reference, Index, acknowledgement, definitions, abbreviations, list of tables, list of figures, and certificates.
- The certificate of plagiarism will be issued by R&D and need to be enclosed in dissertation /thesis/project/internship/clerkship books
Instructions to reduce plagiarisms
-
- Concise the introduction to 10-15 pages only, but focus on importance, need, challenges, risk, applicability, current trends based write up relating the area of your research.
- Definitions and abbreviations may be added as annexure, which do not need plagiarism.
- Compile the content from several resources.
- Include pictures, graph, images wherever possible instead a paragraph explanation.
- Rewrite all text with change in phrase, verb, and sentence forms.
- Literature – do not paste abstract, make it table form, or write 3-5 sentence on each literature.
- Try to arrange flow chart / pert chart based procedure in the methodology/experimental part.
- Result and discussion should be own written.
- Do not copy any content from old research report.
RERDS-CPR Infrastructure
| Name of the facility | Area |
| Director office (RERDS-CPR) /Discussion Room | 430 sq ft |
| Molecular Modelling & Drug Discovery Laboratory | 968 sq ft |
| Sophisticated Analytical Instrumentation Facility | 1022 sq ft |
| Pharmacology & Toxicology Laboratory | 807 Sq ft |
| Phytomedicine & Phytochemistry Laboratory | 807 Sq ft |
| Central facility – DST FIST supported | 807 Sq ft |
| Formulation & Drug Delivery (Pilot Plant ) Laboratory | 914 sq ft |
| CPCSEA Approved Animal House | 753 Sq ft |
| Store Room | 355 Sq ft |
| Library | 600 Sq. Ft |
- Major Equipment /facility – Molecular modelling & Drug Discovery Laboratory
| Name of the facility | Make | Number |
| Molecular Docking software | Schrodinger,
AutoDock, Chimera, Discovery studio |
01
01 01 01 |
| QSAR | Build QSAR | 01 |
| Rotary evaporator | Aditya | 02 |
| Parallel Synthesizer | Radleys 12 plus | 01 |
| Vacuum dryer | Thermo | 01 |
| Hot plates | Remi | 02 |
| Hot air Oven | Remi | 02 |
| TLC plate /assembly | Borosilicate | Adequate |
| Column chromatography for purification | Borosilicate | 02 |
Current Research areas
- Synthesis & Purification of organic molecules
- Study of Physicochemical properties
- Molecular Docking studies
- ADMET prediction – Lead Optimization
- Design of Experiments by Quality by Design
- Major Equipment /facility – Formulation and Drug Delivery (Pilot Plant) Laboratory
| Name of the facility | Make | Number |
| Rotary Tablet Machine (GMP Model) | Rimek | 01 |
| Dissolution Apparatus (Oral Delivery) | Electro Lab | 01 |
| Diffusion cell Apparatus (Transdermal Delivery) | Orchid | 01 |
| Disintegration Apparatus | Electro Lab | 01 |
| Brookfield Viscometer | Amkette | 01 |
| Nano particle and Zeta potential Analyser | Horiba | 01 |
| All-purpose Equipment (Coating, Granulator, Mixer) | Orchid | 01 |
| Moisture Analyser | Fabricated | 01 |
| Tray dryer | Thermo | 01 |
| Double cone blender | Remi | 05 |
| Hot air Oven | Remi | 02 |
| Rota evaporator | Borosilicate | 01 |
| HPLC | Elico | 01 |
| UV-Visible Spectrometer | Shimadzu | 01 |
Current Research areas
- Development and characterization of nano formulations by applying Quality by Design (QbD) principles for various objectives like solubility & stability enhancement of API, targeting to various organs.
- Design and fabrication of various types of tablets to tailor the drug release.
- Design and fabrication of drug delivery systems to treat cerebral malaria and cancer.
- Major Equipment /facility – Sophisticated Analytical Instrumentation Facility
| Name of the facility | Make | Number |
| HPLC (Binary Pump with PDA detection) | Waters | 01 |
| HPLC (Binary Pump with PDA detection) | Agilent | 01 |
| HPLC (Isocratic with UV-VWD) | Agilent | 01 |
| Flash LC System | Analyx | 01 |
| FT-IR (KBR & ATR techniques) | Bruker | 01 |
| GC-FID | Bruker | 01 |
| UV-Visible Double Beam Spectrophotometer | LAB India | 01 |
| ELISA Reader | Thermo Scientific | 01 |
| Lyophilizer | Cintex | 01 |
| Environmental Chamber (Stability Studies) | REMI | 01 |
| Cooling Incubator | REMI | 01 |
| pH meter | Elico | 02 |
| Conductivity meter | Elico | 01 |
| Karl Fisher Auto titrator | Elico | 01 |
| Colorimeter | Elico | 01 |
Current Research areas
- Analytical method development by using HPLC, GC & UV-Vis spectrophotometer.
- Stability studies for pharmaceuticals and dosage forms by HPLC
- Impurity profiling and impurity characterization by HPLC
- Spectral studies by using FT-IR & UV spectroscopy.
- Analytical QbD
- Major Equipment /facility – Pharmacology & Toxicology Laboratory
| Name of the facility | Make | Number |
| Spectrophotometer/Fluorometer DS-11FX+ | Denovix | 01 |
| Semi Auto Analyzer | Erba | 01 |
| Digital Plethysmometer | Inco | 01 |
| Digital Physiograph | Inco | 01 |
| Tissue Homogenizer | Remi | 01 |
| Swim Test Apparatus | Inco | 01 |
| Metabolic cage | Inco | 01 |
| Actophotometer | Inco | 01 |
| Pole Climb Response Apparatus | Inco | 01 |
| Digital Thermometer | Inco | 01 |
| Digital Rotating Drum | Orchid | 02 |
| Digital Organ Bath | Orchid | 02 |
| Centrifuge | Remi | 01 |
| Elevated Plus maze | Fabricated | 01 |
| Hole Board | Fabricated | 01 |
| Stair Case | Fabricated | 01 |
| Y – Maze | Fabricated | 01 |
Current Research areas
- Cancer,
- Neurodegenerative disorders
- Behavioural diseases,
- Gastrointestinal diseases,
- Autoimmune diseases,
- Cardio metabolic syndrome,
- Heamatological diseases,
- Inflammatory diseases,
- Infectious diseases.
- Major Equipment /facility – Phytomedicine & Phytochemistry Laboratory
| Name of the facility | Make | Number |
| Soxhlet Apparatus | Borosilicate | 02 |
| Clevenger’s Apparatus | Borosilicate | 04 |
| Column Chromatography | Borosilicate | 02 |
| TLC | Mason technology | 01 |
| UV Cabinet | Thomas scientific | 01 |
| Rotavap (Shared) | Buchi | 01 |
| Hot air oven | Remi | 01 |
| Flash LC System (Shared) | Analyx one | 01 |
Current Research areas
- Extraction of Phytochemical constituents from plant sources.
- Preliminary identification tests
- Isolation or Separation of active constituents.





